I’m so very, very tired of writing about the devastation that our Secretary of Health and Human Services Robert F. Kennedy, Jr. is wreaking on our federal public health apparatus, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the Food and Drug Administration. On the other hand, it seems well nigh impossible these days to write about some cool science without acknowledging this backdrop, which is one reason why I found a study published last week in Nature fascinating and the reaction to the study by antivaxxers rather typical.
The study is entitled SARS-CoV-2 mRNA vaccines sensitize tumours to immune checkpoint blockade, and its first author is Adam J. Grippin, with its corresponding author being Steven Lin. The key takeaway point from the study is that patients treated with immunotherapy, specifically immune checkpoint inhibitors (ICIs), who had been vaccinated against COVID-19 with one of the mRNA vaccines within 100 days of initiating therapy with ICIs survived longer than those who had not. Looking at this finding in retrospective data, the authors tested the hypothesis that COVID-19 mRNA vaccines sensitize tumors to ICI therapy and found the same effect in mice, allowing them to do additional preclinical studies that suggest a potential mechanism for the effect. If this effect holds up in subsequent studies, it suggests that, far from causing “turbo cancers,” COVID-19 vaccines might actually prime the immune system against “immunologically cold” cancers, an effect that potentiates the action of ICIs.
Before I dig into the study itself, let’s look at ICIs and how they have revolutionized cancer therapy. I’ll use an example from my specialty, breast cancer, to look at how ICIs are now used to treat a specific subtype of breast cancer known as triple-negative.
Immune checkpoint inhibitors
One of the most important discoveries in cancer therapy in the last couple of decades has been the targeting of immune checkpoints with a class of humanized monoclonal antibodies that, as a class, are now referred to as immune checkpoint inhibitors (ICIs). The existence of immune checkpoints was discovered in the 1990s by Tasuko Honjo, and Jim Allison, Ph.D. Allison observed that T-cells are controlled by a safety mechanism or “brake,” specifically a negative immune checkpoint protein called cytotoxic T-lymphocyte–associated protein 4 (CTLA-4). T-cells are usually “turned on” by a cell called an antigen-presenting cell, which has a targeted antigen bound to one of a class of proteins from the major histocompatibility complex (MHC) via the T-cell receptor (TCR). This binding results in the activation of the T-cell to attack cells that have the antigen that had been presented to it, such as a cancer cell. Such an activated T-cell is known as an antigen-specific T-cell.
When CTLA-4, which is found on the surface of T-cells is activated by binding to a protein known as B7, this interaction works to turn T-cell off, preventing it from becoming an antigen-specific T-cell and attacking its targeted cells, including cancer cells, keeping the T-cell in an inactive (non-killing) state. Physiologically, this is useful mechanism to prevent overactivation of the immune system that could cause excessive damage to surrounding normal tissue. Of course, cancers are very good at evolving strategies to subvert natural physiological mechanisms (like angiogenesis—stimulating the ingrowth of new blood vessels to supply their need for oxygen and nutrients—for example) to their own advantage, and, not unexpectedly in retrospect, some cancers have evolved ways of activating these immune checkpoints, thus tamping down the immune response that might otherwise have targeted the invading cancer cells. After his discovery, Allison was able to block CTLA-4, which freed the T-cells stopped from working at the immune checkpoint to resume working normally and eliminate cancer in laboratory mice.
Here’s a useful illustration from the National Cancer Institute:
During the same decade, Honjo was studying what is now the most commonly targeted immune checkpoint inhibitor protein duo, the programmed cell death protein 1 (PD-1)/Programmed Cell Death Ligand 1 (PD-L1) system. In this system, PD-1 is the ICI that is expressed not he surface of T-cells, and PD-L1 binds to PD-1 to inhibit T-cell activation. Again, from the NCI, here’s a diagram showing how this system works and how it can be inhibited:
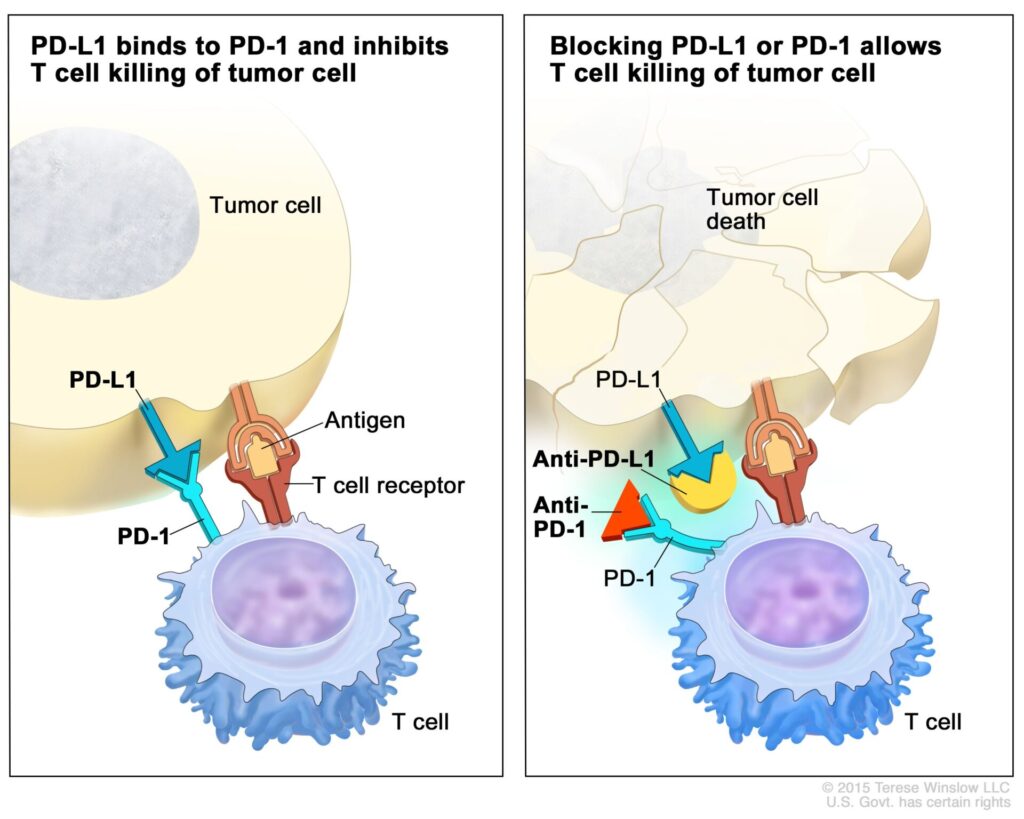
Again, unsurprisingly, some cancer cells express PD-L1, which allows them to activate immune checkpoint inhibition and dial down the T-cell response against them, while blocking the binding of PD-1 and PD-L1, with monoclonal antibodies to either has proven to be an effective strategy to reverse the negative immunomodulation that tumor cells can use to evade the body’s immune responses to them. So important have the discovery of immune checkpoints and how immune checkpoint inhibition can be used to effectively treat a growing number of cancers been to cancer research that in 2018 the Nobel Prize in Physiology or Medicine was awarded jointly to James Allison and Tasuku Honjo “for their discovery of cancer therapy by inhibition of negative immune regulation.”
Of course, my description of how immune checkpoints work and how immune checkpoint inhibitors can be used to treat cancer is, by necessity, very basic. It’s way complicated than the above, but for purposes of my discussion, what I’ve described will have to suffice. After all, thousands of papers and many book chapters have been written about the mechanisms of immune checkpoints and their inhibition, as well as strategies to treat cancer using immune checkpoint inhibition. Since the initial discovery of immune checkpoint inhibitors, immune checkpoint inhibition has been found to be effective in a number of kinds of cancer, including but not limited to:
In my field of specialty, breast cancer, the immune checkpoint inhibitor pembrolizumab (trade name Keytruda) has revolutionized the treatment of the deadly subtype of breast cancer known as “triple-negative” breast cancer (TNBC) as a result of the KEYNOTE-522 trial. In this trial, pembrolizumab was combined with chemotherapy treatment before surgery, the combination resulting in a much higher rate of pathological complete response (pCR, no viable tumor seen in the surgical resection specimen) than standard chemotherapy alone. Indeed, the pCR rate was 65% in the experimental group, which was higher than ever observed for TNBC before. This result translated to a significant improvement in overall survival. After the reporting of the results of KEYNOTE-522 in 2020, almost overnight the standard of care for TNBC changed to immune checkpoint inhibitors plus chemotherapy. This is but one example of the power of ICIs.
COVID-19 vaccination and ICIs
Last week, Adam Grippin and colleagues presented their results at the European Society for Medical Oncology Congress in Berlin, and soon after their results were published in Nature. Although this was a retrospective study and not a prospective randomized controlled trial, the results were striking and very promising. In a commentary in The Scientist Grippin and colleagues explain their rationale in lay language:
The COVID-19 mRNA-based vaccines that saved 2.5 million lives globally during the pandemic could help spark the immune system to fight cancer.1 This is the surprising takeaway of a new study that we and our colleagues published in the journal Nature.2
While developing mRNA vaccines for patients with brain tumors in 2016, our team, led by pediatric oncologist Elias Sayour, discovered that mRNA can train immune systems to kill tumors – even if the mRNA is not related to cancer.3
Based on this finding, we hypothesized that mRNA vaccines designed to target the SARS-CoV-2 virus that causes COVID-19 might also have antitumor effects.
So we looked at clinical outcomes for more than 1,000 late-stage melanoma and lung cancer patients treated with a type of immunotherapy called immune checkpoint inhibitors.4 This treatment is a common approach doctors use to train the immune system to kill cancer. It does this by blocking a protein that tumor cells make to turn off immune cells, enabling the immune system to continue killing cancer.
In brief, Grippin et al carried out a non-interventional, retrospective review of patient data using the MDACC electronic health record system, which contains a record of the patients who are treated at the primary campus of the University of Texas-MD Anderson Cancer Canter, examining patients with melanoma and lung cancer thusly:
The chart review for this study involved three groups of patients: (1) patients with tumour biopsies confirming stage III or stage IV NSCLC [non-small cell lung cancer] between January 2017 and September 2022; (2) patients with melanoma of any stage who received single- or multi-agent immune checkpoint blockade between January 2019 and December 2022; and (3) a tissue-agnostic cohort, which included all patients with pathology results for PD-L1 from January 2020 to October 2023 at our institution across a wide range of histologies.
And:
Patients were separated into two groups: (1) patients who received a COVID-19 mRNA vaccination within 100 days of ICI start; and (2) patients who did not receive a COVID-19 vaccination. Survival analysis was performed using these groups, with subanalysis involving staging of the tumour, brand of mRNA vaccine, number of doses of the COVID-19 vaccine, location of metastases and cycle of immunotherapy.
The authors did their best to control for as many confounders as they could. Indeed, from an interview for Science:
Grippin says he and his colleagues “utilized as many statistical approaches as we could” to account for potential confounding factors, but the association between improved survival rates and COVID-19 vaccines persisted. Patients who received non-mRNA vaccines for influenza and pneumonia, for example, didn’t do better than the average immunotherapy patient.
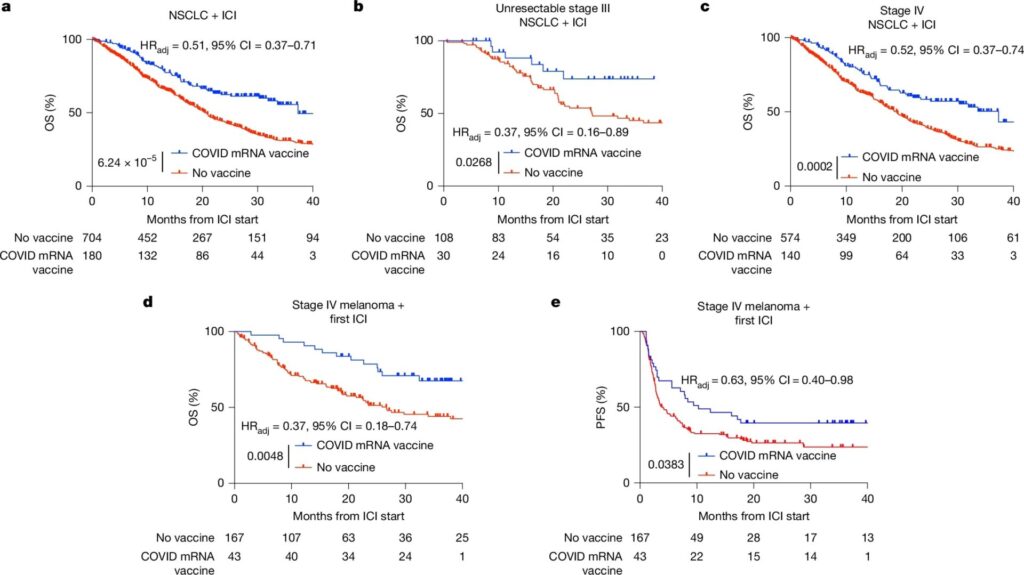
But how much was the increase in overall survival observed in the patients who had received at least one COVID-19 vaccination within 100 days of starting immune checkpoint inhibitor therapy for their cancer? As I like to say, a picture is worth the proverbial thousand words:
Or, to put it in verbiage:
After controlling for 39 covariables with Cox proportional hazards regression, including clinical stage, histology, steroid use, performance status, mutation status, comorbidities and treatment year, we found that receipt of a COVID-19 mRNA vaccine within 100 days of initiation of ICI was associated with significantly improved median OS (20.6 months versus 37.3 months) and 3 year OS (30.8% versus 55.7%, adjusted hazard ratio (HRadj = 0.51, 95% confidence interval (CI) = 0.37–0.71, P < 0.0001) (Fig. 1a and Supplementary Tables 2 and 3). This survival advantage was similar for patients with stage III unresectable NSCLC (HRadj = 0.37, 95% CI = 0.16–0.89, P = 0.0268) (Fig. 1b and Supplementary Tables 4 and 5) and stage IV NSCLC (HRadj = 0.52, 95% CI = 0.37–0.74, P = 0.0002) (Fig. 1c and Supplementary Tables 6 and 7); patients who received mRNA vaccines from either vaccine manufacturer (Extended Data Fig. 1a); and patients who had or had not received a previous COVID-19 mRNA vaccine (Extended Data Fig. 1b). Patients who received two vaccines in the 100 days surrounding initiation of ICI experienced similar OS compared with those who received only one vaccine (Extended Data Fig. 1c). These results were also consistent when considering only those patients whose closest mRNA vaccine was within 100 days before their first ICI (Extended Data Fig. 1d), when narrowing the vaccination window to 50 instead of 100 days (Extended Data Fig. 1e), when restricting to only those patients treated during the pandemic (Extended Data Fig. 1f), after correcting for immortal time bias (Extended Data Fig. 1g) and with propensity score matching (PSM; Extended Data Fig. 1h,i). Patients who received a COVID-19 vaccine within 100 days of chemotherapy (a group that did not include targeted therapies owing to significant heterogeneity and limited patient numbers within different drug cohorts) but did not receive ICI had no detectable survival benefit (Extended Data Fig. 2a). Likewise, patients who received a pneumonia or influenza vaccine within 100 days of initiating ICI (Extended Data Fig. 2b–e) and those with resectable stage III tumours (Extended Data Fig. 2f,g) experienced no improvement in survival.
For advanced stage cancers of this sort, this is a big deal, a very large survival benefit. Basically, the median survival of those who had received a COVID-19 vaccination within 100 days of starting immunotherapy was nearly twice as long as it was for those who had not. It would be easy to dismiss the results as coming from a retrospective study and not an RCT if (1) they weren’t so dramatic; (2) the authors hadn’t controlled for so many confounders; and (3) the authors hadn’t seen similar results from non-mRNA-based vaccines.
Even more interesting is how this result built on previous work in a mouse model by the same group. In their study, published in July in Nature Biomedical Engineering, the authors showed that early interferon responses (interferons are a class of critical immune modulatory proteins) could be enhanced via systemic administration of lipid particles loaded with mRNA coding for antigens not specific to the tumor and that the enhancement could bolster the efficacy of ICIs. (There are other papers suggesting that mRNA vaccines not necessarily expressing a specific tumor antigen can be used to augment cancer immunotherapy.) It was this result that led the authors to ask the question of whether COVID-19 vaccines could have a non-COVID-specific effect that could enhance tumor response to ICI therapy.
Based on the observation in their human retrospective study that administration of COVID-19 vaccines before ICI therapy was associated with a major improvement in survival in advanced NSCLC and melanoma, the authors went back to their mouse models, recreating commercial preparations of COVID mRNA vaccines for administration to tumour-bearing animals in conjunction with ICIs. The authors chose mouse B16F0 melanoma and Lewis lung carcinoma (LLC) as models to test immunogenicity and efficacy of spike RNA-lipid nanoparticle vaccines due to the effects of the vaccine in these clinical settings and because both tumor models are poorly responsive to ICIs. (As a side note, I did a lot of work with Lewis lung carcinoma in mice in the 1990s, for example this paper, and am quite familiar with the tumor model.) Without going into all the experiments they did, I can say that (1) they basically replicated in mice what they had observed in the retrospective study and (2) using a different antigen (in this case, the cytomegalovirus antigen pp65, which is expressed in in human glioma but not in B16F0 tumors) they showed similar results, with the addition of mRNA vaccine to ICI therapy resulting in more potent anti-tumor effects.
As the authors summarized in another article:
We used mouse models to show that mRNA vaccines stimulate inflammation, which drives innate immune cells in the tumour to train other immune cells, called T cells, to infiltrate and kill tumours. Although the tumours counter this by displaying a molecule called PD-L1 that shuts down the immune attack, combining the mRNA vaccine with antibodies to block PD-L1 overcomes this defence mechanism and enables tumour killing. We found similar effects of vaccination in people, including broad immune activation in healthy volunteers and PD-L1 expression on tumour cells in individuals with cancer.
Together, these data indicate that widely available mRNA vaccines might sensitize tumours to immune-checkpoint blockade. The findings challenge the prevailing assumption that mRNA cancer vaccines function mainly by training immune cells to target pre-selected tumour antigens, and introduce another mechanism by which mRNA vaccines can trigger antitumour immune responses. Our data indicate that off-the-shelf mRNA vaccines could be useful in the first-line or pre-surgical therapy setting, and lay the groundwork for the development of universal immune stimulants to sensitize tumours to immune-checkpoint blockade.
Ain’t science cool?
Equally compelling is the potential for turning immunologically “cold” tumors “hot.” A “cold” tumor is a tumor that is unlikely to provoke much of an immune response because they tend to be surrounded by cells that are able to suppress the immune response and keep T cells from attacking the tumor cells and killing them. As the authors write:
Our findings suggest that mRNA vaccines may provide just the spark the immune system needs to turn these “cold” tumors “hot.” If validated in our upcoming clinical trial, our hope is that this widely available, low-cost intervention could extend the benefits of immunotherapy to millions of patients who otherwise would not benefit from this therapy.
Another potential advantage of this approach is this:
We and many others are currently working hard to make personalized mRNA vaccines for patients with cancer. This involves taking a small sample of a patient’s tumor and using machine learning algorithms to predict which proteins in the tumor would be the best targets for a vaccine.5 However, this approach can be costly and difficult to manufacture.6
In contrast, COVID-19 mRNA vaccines do not need to be personalized, are already widely available at low or no cost around the globe, and could be administered at any time during a patient’s treatment. Our findings that COVID-19 mRNA vaccines have substantial antitumor effects bring hope that they could help extend the anti-cancer benefits of mRNA vaccines to all.2
Again, this is the opposite of the nonsensical claim from antivaxxers that COVID-19 vaccines have been responsible for an epidemic of “turbo cancer,” a term never heard or used before COVID-19 vaccines that appears to have been made up of whole cloth by antivaxxers to describe very aggressive cancers that, according to them, were caused by COVID-19 vaccines. Never mind that, even aside from the extreme biological implausibility of any vaccine or exposure causing a rash of cancer diagnoses within a year (which was when the first claims of “turbo cancer” started circulating in the darkest recesses of antivax social media, there is zero evidence to support the claim that the COVID-19 vaccines cause cancer, much less highly aggressive “turbo cancers.”
Here I’ll interject by saying what a lot of scientists have been saying about this study. It’s preliminary. However, it’s also very exciting and compelling, because Grippin et al have combined their retrospective clinical observation with experiments in mice that suggest a plausible mechanism for the effect observed and a rationale to design a randomized controlled clinical trial to test the hypothesis that mRNA vaccines administered to patients with cancers that can be treated with ICIs within 100 days of commencement of therapy will enhance the antitumor effect of the ICIs. Even better, these findings suggest that a strategy in which an mRNA anti-tumor vaccine is developed using an antigen specific to the tumor being treated will be highly effective, because not only would the vaccine provoke an immune response to the tumor antigen but would also have the sorts of nonspecific responses observed by Grippin et al using nRNA-based COVID-19 vaccines.
Unfortunately, despite the promise of mRNA vaccines:
The finding is one more example of the potential of mRNA-based technologies for treating rare cancers and genetic diseases in addition to protecting against infection. Yet political attacks on them, based on unproven safety concerns, are mounting—with some states considering legislation to outlaw mRNA products. In August, Robert F. Kennedy Jr., secretary of the U.S. Department of Health and Human Services, announced the agency was terminating nearly $500 million in grants for research into mRNA vaccines. Treating some applications of mRNA technology as “politically taboo” threatens to create a “chilling effect” for the field, Coller says.
It is because of this “stigma,” Lin explains, that his team hasn’t attempted to seek federal funding for its planned clinical trial. But he remains optimistic that the new findings will attract interest and support from other backers. “There’s so much yet to learn,” he says. “This is just the beginning.”
Steven Lin is the corresponding author and principal investigator who oversaw this research.
In the beforetime (before Trump and HHS Secretary RFK Jr.), NIH funding for such a clinical trial would have bene virtually assured, given the rigor of the science and the promise of the findings reported last week. Now, with mRNA vaccines being demonized and the mRNA platform being falsely portrayed as dangerous by the “make America healthy again” (MAHA) activists now running all non-military federal health programs, Dr. Lin is forced to beg for scraps to fund what is likely to be a highly impactful clinical trial that could revolutionize cancer therapy as much as the discovery of immune checkpoint inhibitors did over the last 20 years and angiogenesis inhibitors did the decade before that.
Fortunately, for the world, the US isn’t the only country in the world, and the work is being pursued in a number of countries, as described in this Washington Post article:
“It is not unexpected. You can expect that more data will come out,” said Katalin Kariko, the University of Pennsylvania researcher who shared the 2023 Nobel Prize in medicine with Drew Weissman for work that led to the development of the coronavirus vaccines.
Kariko, who was not involved in the new study, said that in May she was in Europe speaking with other scientists “and they mentioned that the covid vaccine has an effect on cancer growth.” There are about 150 clinical trials of mRNA vaccines ongoing around the world, Kariko said, almost half for treatment of infectious diseases and many of the others for cancers.
The damage that President Trump and his lackey HHS Secretary RFK Jr. have done to public health, medicine, and biomedical research in the US has already been monumental. The damage is so severe that the CDC is in free fall and irreparably damaged, thanks to massive layoffs and budget cuts, and the NIH and FDA are barely functional. Meanwhile, NIH and other government funding for one of the most promising medical technologies yet devised for vaccines and to treat cancer, mRNA delivered by lipid nanoparticles, has been eliminated, all because of the delusional beliefs of antivaxxers that somehow mRNA technology is evil and harmful.
When the supremacy of the US in biomedical research is surpassed by China and the European Union, something that is likely to happen in the next few years at the most and the brain drain of young scientists who see the writing on the wall and decide to depart for greener pastures leaves us a distant third (if even that) in biomedical research, it will have been an entirely self-inflicted wound, one we as a nation will probably never recover from. Foregoing studying the promise of mRNA vaccines as cancer treatments are just one example; there are, sadly, many, many more.

